Vinyl chloride or allyl chloride.
Vinyl chloride friedel crafts.
Reactions on a solid surface.
The reaction was stirred for 15 min.
The friedel crafts alkylation involves the electrophilic substitution of alkyl groups on aromatic rings when arenes are treated with alkyl halides in presence of lewis acids.
Friedel crafts alkylation was first discovered by french scientist charles friedel and his partner american scientist james crafts in 1877.
The friedel crafts reaction of 1 phenylthio vinyl chlorides 1 with arenes proceeded in the presence of alcl3 or etalcl2 to give 1 arylalkenyl sulfides 2.
Typical experimental procedure for friedel crafts cyclization of vinyl chloride to afford 3 chloro 1 naphthol 2.
After 10 min aluminum chloride 2 mmol was added.
This reaction is catalyzed by lewis acids like anhydrous alcl 3 fex 3 zncl 2 bf 3 etc.
A simple economical and efficient friedel crafts acylation reaction over zinc oxide zno as a new catalyst m.
According to me vinyl chloride will be a better electrophile than allyl chloride hence allyl chloride should be the answer.
This reaction allowed for the formation of alkyl benzenes from alkyl halides but was plagued with unwanted supplemental activity that reduced its effectiveness.
Mild efficient friedel crafts acylations from carboxylic acids using cyanuric chloride and alcl 3.
The alkenes or alcohols can also be used to alkylate aromatic rings under friedel crafts conditions.
The reaction between.
The friedel crafts reaction of 1 phenylthio vinyl chlorides 1 with arenes proceeded in the presence of alcl 3 or etalcl 2 to give 1 arylalkenyl sulfides 2 the stereoselectivity indicated that the reaction is a kinetically controlled process.
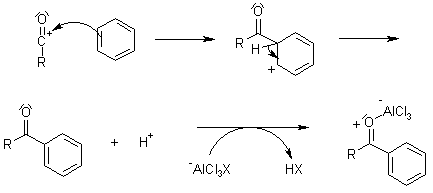
In a friedel crafts acylation reaction the aromatic ring is transformed into a ketone.
At rt to acid 1 mmol in ch 2 cl 2 5 ml was added two drops of dmf and oxalyl chloride 2 mmol slowly.
For primary and possibly secondary alkyl halides a carbocation like complex with the lewis acid r x mx n is.
Which of the following compounds will not undergo friedel crafts reaction with benzene.
The friedel crafts acylation reaction involves the addition of an acyl group to an aromatic ring.
The stereoselectivity indicated that the reaction is a kinetically controlled process.
Chem 2004 69 6953 6956.